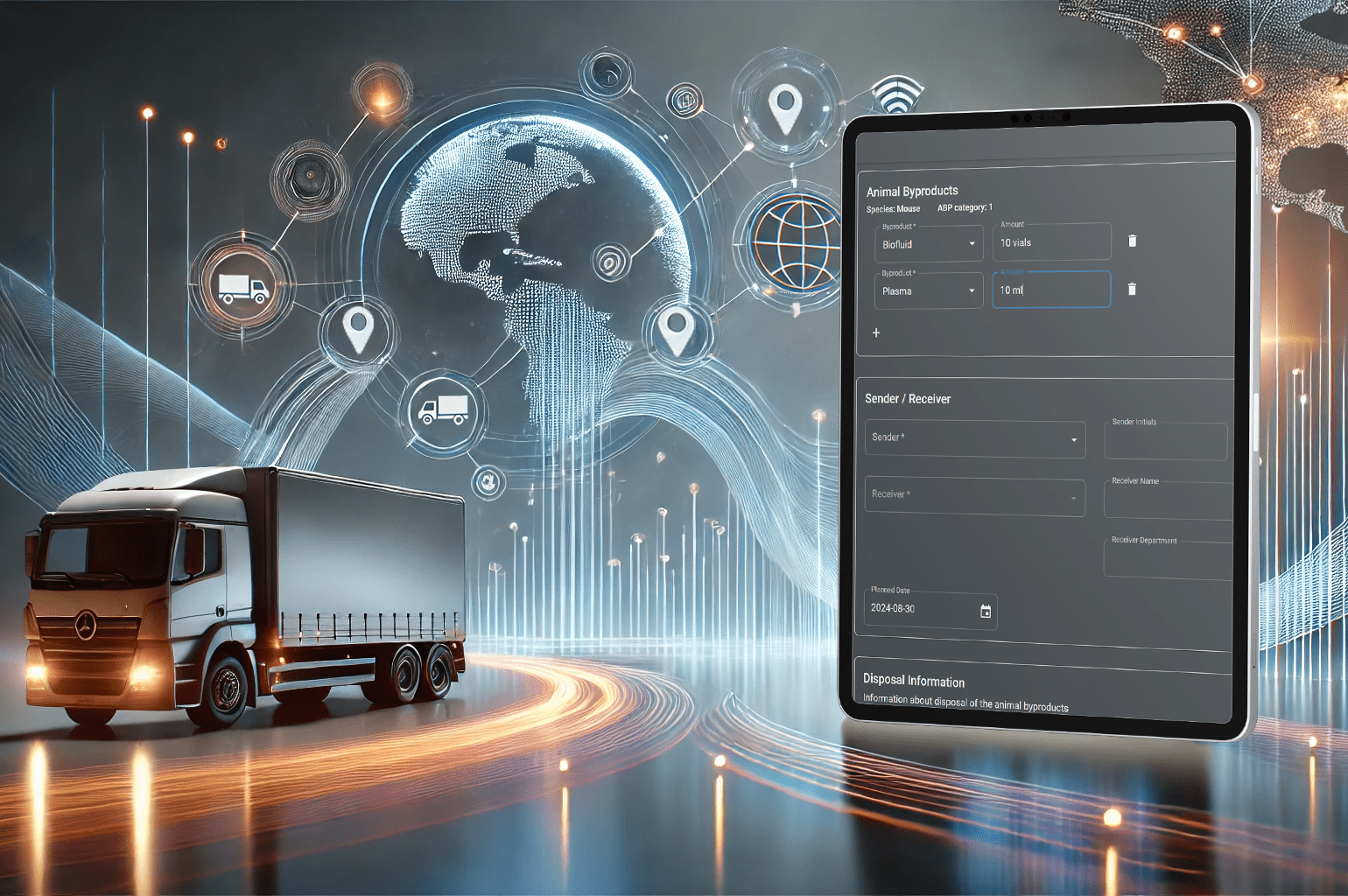
A case study showcasing the digital traceability system’s features: real-time tracking, risk categorization, and digital record-keeping
Background
In an animal research facility, managing animal by-products is essential for regulatory compliance and public health. EU Regulations 1069/2009 and 142/2011 require strict traceability to prevent health risks and ensure safe handling. Previously, the facility relied on manual processes that were time-consuming and prone to errors, making quick responses challenging.
To improve efficiency, the facility implemented Picoteam’s Digital Traceability System, which provides real-time data access and tracks biological materials from origin to disposal. The system categorizes materials by risk level to meet EU standards and digitalizes documentation for easy retrieval during inspections. With instant access to critical information, the facility can respond swiftly to health risks, strengthening both compliance and safety.
How the System Changed Daily Operations
Picoteam’s Digital Traceability System has noticeably improved the facility’s daily operations, making processes more efficient and enhancing compliance. By replacing manual methods, the system simplifies document management, improves inventory accuracy, and centralizes tracking. Staff can now follow safety protocols with ease.
Digital Commercial Documents and Records
The shift to digital document storage eliminates the need for managing extensive physical paperwork. This change has significantly eased the audit process, as documents are now accessible on multiple devices at any time, making preparation and retrieval quicker and more efficient. This streamlined approach enables facilities to promptly verify traceability information, enhancing compliance and operational efficiency.
Real-Time Inventory System
The facility’s inventory management has been improved by real-time tracking. Staff have instant access to up-to-date inventory levels, minimizing the errors that came with manual logging. This feature has made it easier for personnel to verify stock and meet compliance requirements, reducing the risk of overlooked materials.
Seamless Integration with Existing Data
Picoteam’s solution integrates with existing data systems, enabling a smooth transition without the need for re-entering data. This continuity ensures that historical records are preserved, enhancing traceability and minimizing disruption. Staff can continue working with familiar data sets, maintaining productivity and operational efficiency throughout the shift to digital traceability.
Dispatching and Receiving Registries
The digital registries for incoming and outgoing materials have centralized all tracking information. Staff no longer need to rely on separate logs or physical documentation, as every movement of material is now documented in one place. This centralization has improved accountability and reduced the risk of lost or inaccurate records, making compliance much simpler.
Permission Registry
With an integrated permission registry, access to permission information for senders, receivers, and transporters is ensured, verifying that they have the appropriate permissions to handle biological materials. This digital authorization check has streamlined safety protocols, allowing staff to quickly verify permissions without additional manual checks, reinforcing both safety and efficiency.
Comprehensive Digital Record-Keeping for Compliance
Maintaining complete digital records has simplified regulatory compliance. Staff now have instant access to records for any stage in the material handling process, making data retrieval for audits straightforward and efficient. This has reduced the time spent on compliance tasks and ensured accurate, up-to-date records at all times.
Risk-Based Categorization
Regulations mandate that animal by-products be categorized by risk level, which determines handling and disposal protocols. Picoteam’s system automates this categorization process, ensuring that materials are handled according to regulatory standards and reducing human error.
Real-Time Monitoring
Real-time tracking enables facilities to monitor the movement of animal by-products across different locations. This capability is crucial for managing potential health risks, as it provides up-to-date information on the location and status of materials.
Results
Since adopting Picoteam’s Digital Traceability Solution, the facility handling animal by-products has seen remarkable improvements in efficiency and compliance. The transition from manual to digital records has reduced administrative burdens, allowing staff to focus on essential tasks. During a recent compliance audit by the authorities—previously a challenging and time-consuming process—the facility passed with flying colors without any preparation work, thanks to the system’s real-time data access and streamlined documentation. This transformation has not only eased audit processes but also strengthened the facility’s compliance standards, demonstrating the value of digital solutions in advancing public health and operational efficiency in animal research